Introduction to Liposome
Liposomes and lipid nanoparticles (LNP) are commonly used as targeted formulations to direct therapeutic drugs specifically to the site where they need to work, with little or no interaction with non-target tissues. Targeted formulations are increasingly gaining widespread attention in the pharmacy community for their ability to increase drug efficacy and reduce toxicity while improving drug safety, efficacy, reliability and patient compliance.
Liposomes were first discovered under a microscope in 1961 by scientist Alec Dougla. Bangham and R. W. Horne. Liposomes are vesicular structures composed of lipid molecules with a lipid bilayer forming a hydrophobic outer shell and an inner aqueous-phase cavity, which is both hydrophilic and hydrophobic.
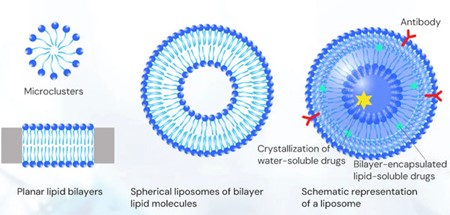
Lipid nanoparticles are nanoparticles formed using a type of lipid. In the past, researchers typically used lipid nanoparticles to directly encapsulate chemical drugs. In the field of gene therapy, researchers have begun to utilize lipid nanoparticles to encapsulate nucleic acids, such as mRNA, siRNA, pDNA, etc., which are called nucleic acid lipid nanoparticles.
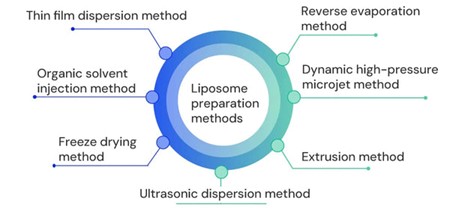
Liposome Preparation Method
The liposome preparation methods are shown in the below graph. The current dynamic high-pressure microjet method produces more uniform liposomes.
Liposome Processing
During liposome processing, small unilamellar vesicles (SUV), large unilamellar vesicles (LUV), Multilamellar large vesicles (MLV) and Multivesicular vesicles (MVV) are produced. The ideal state is small unilamellar vesicles, and other types of liposomes are impurities in the product.
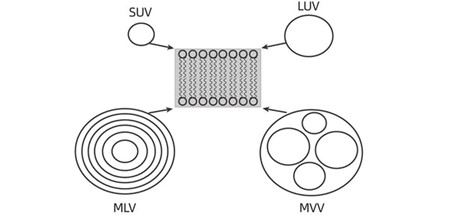
Small unilamellar vesicles (SUV): 20-80nm
Large unilamellar vesicles (LUV): 100-1μm
Multilamellar large vesicles (MLV): 1-5μm
Multivesicular vesicles(MVV): 1-5μm
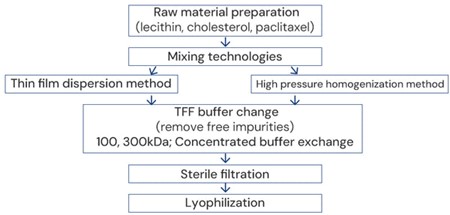
Example: Process route for the preparation of paclitaxel liposomes
Challenges of Liposome Sterile Filtration
Sterile filtration of liposomes presents significant challenges throughout the filtration industry. Due to the nature of the lipid bilayer of liposomes, challenges are posed to the filtration flow rate, loading capacity and bacterial retention capacity of sterilizing filters.
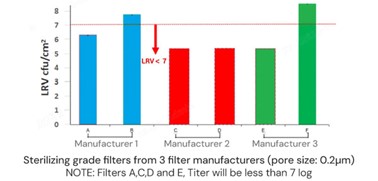
The following is the sterile filtration performance data of three brands of PES/PVDF filters at a constant pressure of 2 bar. (A/B from manufacturer 1, C/D from manufacturer 2, E/F from manufacturer 3), please refer to it.
Cobetter’s Answer to the Challenges
Cobetter selected four PES sterilizing filters (PES-1 to PES-4 membrane). These membranes vary in pore size, structure, and hydrophilicity; the other materials were PVDF sterilizing filters (PVDF membrane), nylon 66 (N66) sterilizing filters (N66-1 and N66-2 membrane, the charging properties of the filter membranes are different), Hydrophilic PTFE sterilizing filter (PTFE membrane). Then we conduct the filterability test and bacterial retention test at a constant pressure of 2 bar.
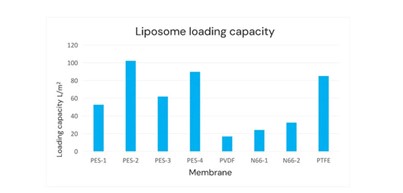
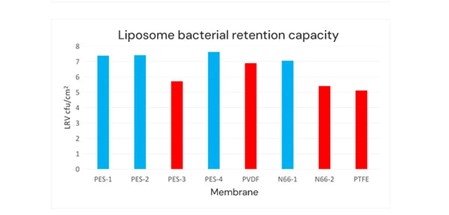
Based on the above filterability test results and bacterial retention test results, the red columns indicate that these sterilizing filters cannot meet the sterilizing requirements for liposome bacterial retention.
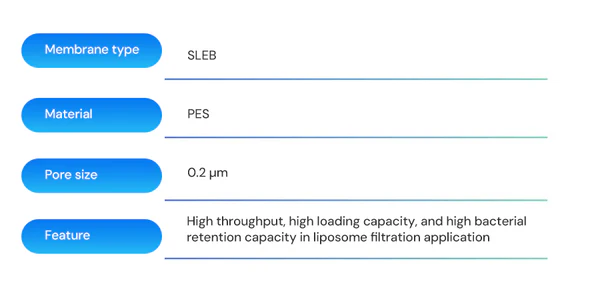
The product with the best performance at present is the PES-2 membrane, which is Cobetter’s SLEB sterilizing membrane composed of a double-layer 0.2μm configuration. It has been validated to have a filtration time of 12 hours. The membrane has a bacterial retention capacity LRV ≥ 7 per square centimeter of effective filtration area with downstream sterility, which highlights the membrane’s application advantages in liposome sterile filtration.