What is PUPSIT (Pre-Use/Post Sterilization Integrity Testing).
The integrity of the sterilized filter assembly should be verified by integrity testing before use (pre-use post sterilization integrity test or PUPSIT), to check for damage and loss of integrity caused by the filter preparation prior to use.
A sterilizing grade filter that is used to sterilize a fluid should be subject to a non-destructive integrity test post-use prior to removal of the filter from its housing. The integrity test process should be validated and test results should correlate to the microbial retention capability of the filter established during validation.
PUPSIT is a test that is used to ensure the integrity of a sterilizing filter and its associated components, such as the filter housing, support structure, and connections, after the filter has been installed and sterilized but before it is used to filter product. PUPSIT is a discussed new requirement in new EU GMP Annex 1.
Global Regulatory Requirements for Integrity Testing:
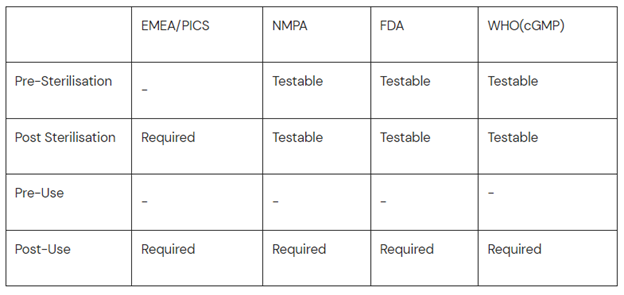
EU GMP: The Integrity of the sterilized filter should be verified before use and should be confirmed immediately after use by an appropriate method such as a bubble point, diffusive flow or pressure hold test.
US FDA: Integrity testing of the filter can be performed prior to process and should be routinely performed post-use.
NMPA: Immediately after use, the integrity of a sterilizing filter must be checked and documented using an appropriate method. Commonly used methods are the bubble point test, diffusion flow test, or pressure hold test.
GMP Guidelines: Integrity testing of finished sterilization grade filters before and after use is a critical element of sterility assurance.
2017 PIC/S guidelines (for PIC/S member countries OR countries who export to PIC/S members): 113. The Integrity of the sterilized filter should be verified before use and should be confirmed immediately after use by an appropriate method such as a bubble point, diffusive flow or pressure hold test.
Cobetter PUPSIT Assemblies
Advantages:
Cobetter PUPSIT system is an assembled piping system that can do in-line integrity testing for redundant sterilization filters or single/two stage sterilization filters pre-use/post sterilization, no need for displacement testing or remote testing, ensuring the system remains sterile before and after filter use.
In addition to a wide range optional of sterilizing filters, the entire system combines customer actual process with Cobetter’s competitive single-use products portfolio, including single-use bags, capsule filters, pressure gauge fittings, in-line pressure sensors, branch fittings, and self-manufactured pipes and tubes. Cobetter can provide customers with flexibility to customize their PUPSIT system design.
According to the different conditions of each customer’s production plant, the design of PUPSIT system is adjusted to be combined with the workshop equipment layouts, and the location of the purified water source, compressed air source, integrity tester and wall pass-through component etc. The performance of the filters, integrity test methods and parameters, as well as the overall situation of the pre-wetting process, etc, are taken into consideration as well. Cobetter aims to provide the customer with reasonable, compliant, easy-to-use and easy-to-operate PUPSIT system solutions.
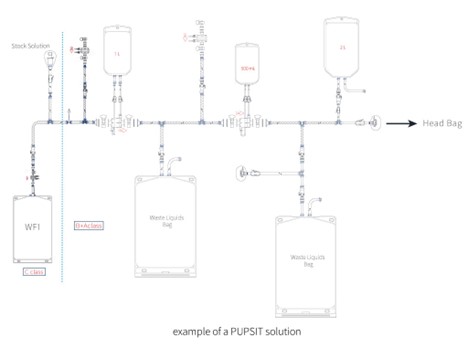
Example of A PUPSIT Solution:
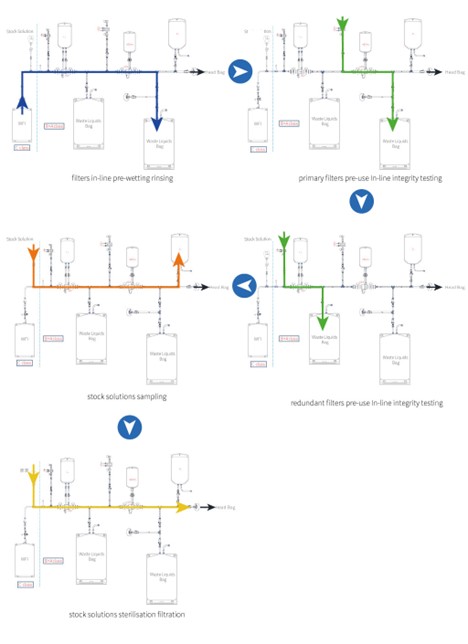
The following is an example of a PUPSIT solution for stock solutions sterilization filtration. Of course, the design of the PUPSIT system is much more than this, the design will also be adjusted and matched in details to the filter wetting conditions, rinse volume, preset room cleanness level, residual liquid collection, final filling and more.
Operating Steps:
Cobetter PUPSIT system comes with clear instructions and is easy to operate. The usual operating steps are as follows: